The sulfur content in the sulfur/carbon composite cathodes mostly appears in the form of the most stable polymorph of sulfur at room temperature, i.e. orthorhombic α-sulfur. During discharge, different surfaces of α-sulfur crystal are exposed to lithium cations (Fig.1). In a conventional Li-S battery with orthorhombic α-sulfur molecular crystal as cathode, reduction of sulfur occurs through sequential lithiation during discharge cycles, S8 → Li2S8 → Li2S6 → Li2S4 → Li2S. The actual lithiation mechanism of the sulfur cathode is dependent on many factors, such as cathode morphology, electrolyte composition, etc. Therefore, it is only partially known so far. One can, however, obtain valuable insights by theoretically predicting the structural changes in the cathode during the discharge (Fig. 2 and 3). Equipped with finite-temperature quantum-mechanical spectroscopy simulation techniques (IR, Raman, NMR, etc.), [1-3] and in a close collaboration with the experimental research groups at the MLU Halle-Wittenberg as well as Helmholtz-Zentrum Berlin, we are now able to compare and validate our theoretically predicted structures for the lithiated cathodes with the spectroscopy measurements.
Structural flexibility of polymeric sulfur materials can withstand the volume expansion of the sulfur cathode upon lithiation. It has been shown that these
MARTIN-LUTHER UNIVERSITÄT
HALLE-WITTENBERG
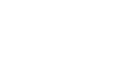

Research
Introduction: Lithium-sulfur batteries
Sulfur with its high theoretical lithium capacity of 1675mAh/g is an abundant and low-cost element. For these reasons, lithium-sulfur (Li-S) batteries are among the most promising energy-storage devices for various applications, such as electric vehicles. During the discharge of a Li-S battery electrochemical dissolution of lithium from metallic Li anode and lithiation of sulfur at the sulfur cathode occurs in an ionic-liquid electrolyte. However, low sulfur utilization and poor cycle life are among issues limiting commercialization of the Li-S batteries. These problems are mainly related to
-
(i)formation of insulating layer composed of Li2S2 and Li2S insoluble structures on the S cathode during the discharge, which results in poor utilization of the active-material;
-
(ii) dissolution of Li-polysulfides (Li2Sx, 2≤x≤8) into the electrolyte before full reduction to Li2S, which leads to irreversible capacity fade;
-
(iii) migration of the Li-polysulfides to the anode (shuttle effect), their reaction with the lithium atoms and eventually formation of an insulating layer of Li2S2 and Li2S around the anode. This layer can obstruct the diffusion of Li+ ions into the electrolyte; and
-
(iv) volume variation of the S cathode upon reaction with Li cations, which is related to different densities of orthorhombic α-sulfur (2.07 g cm-3) and Li2S (1.66 g cm-3). This not only results in loosened electrical contacts with the conductive substrate, but can also lead to safety problems.
Our research manly aims at finding novel sulfur-based cathode materials for the Li-S batteries which mitigate the shuttle effect by either avoiding the formation of Li-polysulfides or by immobilizing them using protecting layers and/or well-tuned electrolyte compositions.
Figure 1: Schematic of a typical sulfur/carbon composite cathode. Lithium and sulfur atoms are shown in purple and yellow colors, respectively. Gray stripes denote representative carbon mesh. Sulfur has been mostly observed in its orthorhombic α-sulfur polymorph exhibiting different surfaces of the molecular crystal.
Sulfur cathode materials
Lithium cation diffusion
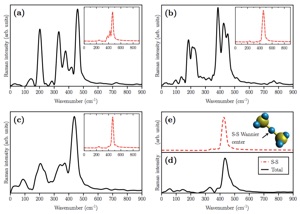
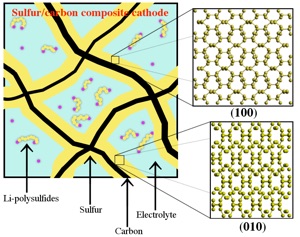
Figure 2: Simulated finite-temperature Raman spectra of (a) (Li2S4)1, (b) (Li2S4)4, and (c) (Li2S4)8 Li-polysulfide clusters. The insets show the contribution of S–S stretching vibration to the total Raman spectra. (d) Simulated Raman spectra of (Li2S2)8 cluster, and (e) locally decomposed Raman spectra for the S–S stretching vibration shown in the inset.
cathode materials also exhibit a promising performance in terms of specific capacity and cycling stability.[3] Generally, the structural complexity of such sulfur-based polymers is very high. For example, polyacrylonitrile-based (PAN) carbon nanofibers can be experimentally realized in various widths, which can substantially change their interaction with their surroundings.[4] Theoretical simulations hint at a considerable hinderance of shuttle effect in the Li-S batteries with sulfur/PAN-based cathodes.[5]
Figure 3: (a) Proposed atomic configuration of the Li2S/carbon nanofiber samples after full lithiation (discharge). Structure of the fibers in early discharge stages (before lithiation) is shown in (b) along with the corresponding simulated partial Raman spectra. Different curves correspond to the partial Raman spectra arising from different fragments of the system which are highlighted by different colors. H, C, N, Li, and S atoms are shown in white, grey, blue, purple, and yellow colors.
References
[1] P. Partovi-Azar and T.D. Kühne, J. Comput. Chem. 36, 2188 (2015).
[2] P. Partovi-Azar, T.D. Kühne, and P. Kaghazchi, Phys. Chem. Chem. Phys. 17, 22009 (2015).
[3] A. Hoefling, D.T. Nguyen, P. Partovi-Azar, D. Sebastiani, P. Theato, S.W. Song, Y.J. Lee, Chemistry of Materials 30, 2915 (2018).
[4] P. Partovi-Azar, S.P. Jand, P. Kaghazchi, Phys. Rev. Applied 9, 014012 (2018).
[5] C.-J. Huang, J.-H. Cheng, W.-N. Su, P. Partovi-Azar, L.-Y. Kuo, M.-C. Tsai, M.-H. Lin, S.P. Jand, T.-S. Chan, N.-L. Wu, P. Kaghazchi, H. Dai, and B.-J. Hwang, J. Power Sources Submitted (2020).
[6] P. Partovi-Azar and D. Sebastiani, Batteries & Supercaps 2, 695 (2019).
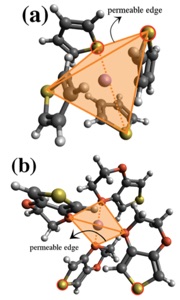
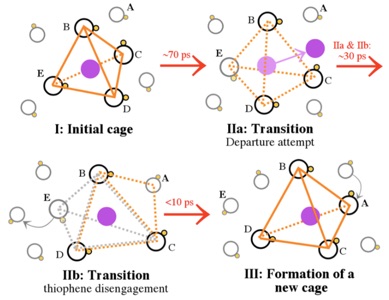
Studying the diffusion mechanism of Li cations in different electrolyte compositions (i.e. solvent + Li-salts) and through cathode-protecting layers (which are sometimes used to limit the diffusion of the Li-polysulfides to regions around the sulfur cathode) is also at the center of our research. Our theoretical investigations include a wide variety of methods, such as ab initio and classical molecular dynamics simulations together with multi-scale modeling techniques to reach realistic time and length scales. Finding electrolyte compositions and/or materials which facilitate the diffusion of Li cations around the sulfur cathode can ultimately lead to design of more efficient Li-S batteries. However, to acquire a reliable picture of the diffusion mechanism sometimes dynamics simulations based on quantum chemistry methods are required. Figure 4 shows the local structure around the Li cation in thiophene (a) and 3,4‐ethylenedioxythiophene (b) liquids as well as the mechanism of cation hopping between thiophene cages, as revealed by our ab initio molecular dynamics simulations.[6]
(c)
Figure 4: Local structure around a Li cation in thiophene (a) and 3,4‐ethylenedioxythiophene (b) liquids. Also shown in (c) is the mechanism of cation hopping between thiophene cages (cage hopping), as revealed by AIMD simulations. H, C, N, Li, and S atoms are shown in white, grey, blue, purple, and yellow colors.

Updated: Mar. 2022
